That share may continue to climb as adults and children age 12 and older receive doses of the coronavirus vaccine. In Travis County, more than half of all residents age 12 and older are fully vaccinated against COVID-19, according to data from the Texas Department of State Health Services. Neighboring Hays and Williamson counties are not far behind.
According to data collected by the American Academy of Pediatrics, up to 2.1% of all COVID-19 cases in children resulted in hospitalization in the week ending May 20. Though COVID-19 hospitalizations are less frequent for children than adults, they can still happen.
Jennifer Macklom, a local school teacher and parent, is enrolling her three young children in an upcoming Austin Regional Clinic Pfizer COVID-19 vaccine trial specifically to help prevent one of her kids from having to face hospitalization. Macklom said she has already faced that experience when one of her children contracted other coronaviruses as a newborn.
“It is still in my mind—all I can see is one of my kids in a hospital bed. If I can make them safer or keep them from getting a bad case of COVID-19, I’m going to do it,” Macklom said.
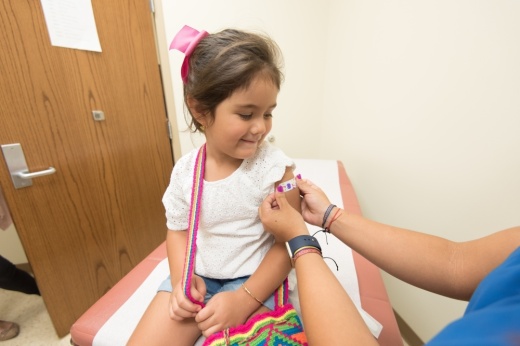
Next month, ARC will start clinical trials to test the safety and efficacy of the Pfizer coronavirus vaccine on children aged 6 months to 11 years old. These trials represent one of the final steps for the vaccination to receive approval from the federal government for distribution. Dr. Jacques Benun, a pediatrician at ARC, said hundreds of families have already signed their children up to be part of the study. Beginning June 7, ARC will start the vaccine trial with 25 children, though that number may grow as Pfizer opens up the vaccine trial to more patients.
The trial itself will last about 6 months as patients come in for their two doses of the vaccine. The second dose is scheduled for 3 weeks after the initial shot. Following the inoculations, Benun said the ARC research team will follow up with the children involved in the trial to draw blood samples and perform analysis.
“The follow-up visits are in-person or by phone [and look] at any side effects to understand what they are in little children, compared to other ages,” Benun told Community Impact Newspaper. “[We are] also looking to see if the vaccine works—if it learns to make antibodies.”
ARC will look for side effects in the patients that adults and older children have experienced so far with the Pfizer vaccine, such as soreness in the arm, fever, chills and body aches, according to Benun.
This study is a blinded experiment, meaning 2 of every 3 children participating in the study will receive the Pfizer vaccine, while the third child receives a placebo. Six months after the second dose is administered to patients in the study, all participants will learn if they have received the vaccine or the placebo. According to the ARC website, participants who received the placebo will have the option to receive the COVID-19 study vaccine at the end of the trial period.
Benun believes the Pfizer vaccine may receive approval for distribution from the U.S. Food and Drug Administration sometime in fall 2021. ARC previously participated in the Pfizer COVID-19 vaccine trial for 12-to-15 year old children, and that vaccination was given federal approval on May 10. The first approved COVID-19 vaccination for adults 16 years old or older was given the green light for distribution in December 2020. When the Pfizer vaccine for young children does eventually get approved, Benun encourages parents to acquire the shot for their kids as soon as they can, saying young children are particularly at-risk to contract COVID-19.
“Children are in environments at school and daycare that they are very close to each other. It is so much easier for them to get COVID-19. This vaccine will offer that protection,” Benun said. “Children can get COVID-19 just like adults. They need to be protected as well.”