Q&A with Dr. Maria Elena Bottazzi:
Dr. Maria Elena Bottazzi, associate dean of the National School of Tropical Medicine at Baylor College of Medicine and co-director of the Texas Children’s Hospital Center for Vaccine Development, spoke with Community Impact Newspaper about vaccine development and the medical community’s response to the coronavirus outbreak. This interview has been edited for length and clarity.What is the outlook on the production of a COVID-19 vaccine?
I think it’s clear that there are many vaccines that are hopefully going to be evaluated soon. ... It’s good to have a robust pipeline, meaning we’re not just assuming that one [vaccine] is going to be the only one that’s going to be a solution for all. Vaccines certainly have a high rate of failure due to the nature of how you develop them and what the expectation of a vaccine [is], which of course is a preventative measure.
How much is the testing phase expected to be sped up now?
Because it’s not business as usual, the regulatory bodies are evaluating how they can allow certain things to maybe be done in parallel rather than sequentially. ... It’s going to be very difficult to skip a step. I don’t think that’s really going to be able to be done, but at least steps can be shortened by sharing the information just in time even though there are risks.
What are some risks associated with accelerated vaccine development?
You are pressuring those who have to evaluate to make decisions that in a normal way of business you wouldn’t because you have more time. ... I trust 100% [in] our regulatory bodies. The people who conform the teams within the [U.S. Food and Drug Administration], for example, are teams that operate with their mission to ensure that their decisions are based on robust and strong scientific evidence. Not hearsay, not because they have to go fast. So I trust that process and that system. It’s challenging that they have to make this in an accelerated manner, but I think we as people have to trust at some level that they’re experts.
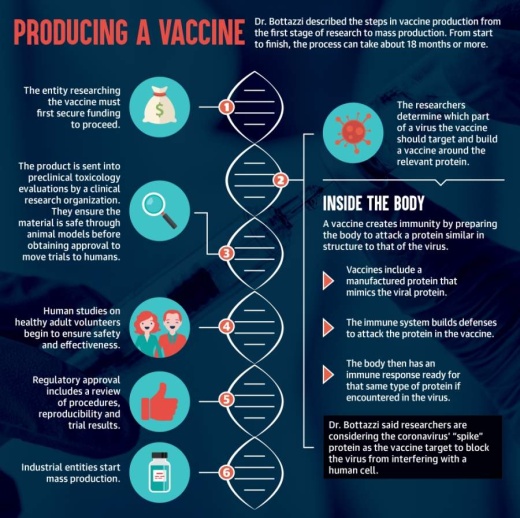
How are vaccines produced?
The first thing you need to do is identify what within the pathogen is important enough you want to interfere with. ... Everyone is talking about interfering with the virus to be able to enter the human cells. And therefore we know the element within the virus that is essential for this activity to happen is this “spike” protein. ... A very small domain of that spike protein [is] enough for us to build a vaccine. And then what you do is you replicate that protein. ... When your body sees this foreign protein, the body knows it’s a foreign entity, and then we have an immune response. ... When you vaccinate, you’re vaccinating with something that resembles a very important piece of the pathogen. ... We then eventually get the virus, we remember they’re a foreign entity, and we already have an army ready to respond.
Local innovations
The Woodlands company VGXI is a contract manufacturer for DNA plasmids, and it manufactured a DNA coronavirus vaccine for Inovio Pharmaceuticals the company said was in human trials this spring.VGXI Inc. announced April 6, it had completed the manufacturing process for its COVID-19 DNA vaccine.
The Woodlands-based plasmid DNA manufacturer first announced it was producing a vaccine for the new coronavirus in late January, after it was selected by Inovio Pharmaceuticals as a production partner under a $9 million grant from the Oslo-based Coalition for Epidemic Preparedness Innovation.
Inovio said its Investigational New Drug application for the vaccine had been approved by the U.S. Food and Drug Administration, and the first phase of clinical testing began April 6 at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia and the Center for Pharmaceutical Research in Kansas City, Missouri. VGXI said Inovio is also coordinating clinical trials in China and South Korea.
VGXI said thousands of vaccine doses were produced within two months, up to four times faster than typical plasmid DNA production. The company scheduled large-scale manufacturing of the vaccine in the second quarter this year, and Inovio said it plans to have 1 million doses available by the end of 2020 for further trials.